Table of Contents
What is Mercuric Chloride
The compound Mercuric chloride, also known as Mercury (II) chloride, Mercury bichloride or Dichloromercury, is a mercury-chlorine compound with the formula HgCl2. It is a crystalline white solid used as an experimental reagent. Mercury chloride is a very highly toxic compound that decomposes slowly at room temperature and rapidly at 100 degrees Celsius. It is a mucous membrane corrosive topical antiseptic and disinfectant. Arab doctors used mercury chloride to disinfect wounds in the Middle Ages, but modern medicine considers it dangerous.
This substance is a transition metal chloride, which is an inorganic compound. It is mainly used as a catalyst for the conversion of acetylene to vinyl chloride, the precursor to polyvinylchloride.
Chemical structure and properties
| PROPERTIES OF MERCURIC CHLORIDE | |
|---|---|
| IUPAC Name | dichloromercury |
| Synonyms | Mercury bichloride; Mercury dichloride; Corrosive sublimate; Mercury perchloride; Sublimat; Chlorure mercurique; Sulema |
| CAS No. | 7487-94-7 |
| Molecular Formula | HgCl2 |
| Molar Mass | 271.5 g/mol |
| Appearance | Colorless rhombic crystals or white powder |
| Odor | Odorless |
| Solubility | Soluble in water, acetone, ethyl acetate, glycerol, ethanol, ether, methanol, and acetic acid |
| Melting Point | 277°C |
| Density | 5.4 g/cm3 (at 25°C) |
| PubChem CID | 24085 |
| PubChem SID | 462770295 |
Synthesis of Mercuric Chloride

Mercuric chloride is formed when chlorine reacts with mercury or mercury chloride (I). Also, Hydrochloric acid can be mixed with a hot, concentrated solution of mercury (I) compounds such as nitrate to obtain Mercuric chloride.
Hg2(NO3)2 + 4 HCl → 2 HgCl2 + 2 H2O + 2 NO2
Another synthesis method includes heating a mixture of solid mercury(II) sulfate and sodium chloride. Mercuric chloride obtained through this method is volatile in nature and can be separated by sublimation.
Uses (Applications)
As a catalyst
Mercuric chloride is primarily used as a catalyst in the conversion of acetylene to vinyl chloride and polyvinyl chloride precursors.
C2H2 + HCl → CH2=CHCl
As a chemical reagent
Amalgams with metals like aluminium are sometimes made with Mercuric chloride. After being treated with an aqueous solution of mercuric chloride, aluminium strips are quickly covered by a thin layer of amalgam. Aluminium is essentially inert
due to a thin layer of oxide that protects it. Once amalgamated, aluminium can go through a variety of reactions. The exposed aluminium, for example, reacts with water to produce Al(OH)3 and hydrogen gas when the oxide layer is removed. Halocarbons react with amalgamated aluminium in the Barbier reaction. Amalgamated aluminium is also used as an agent to reduce organic synthesis. Zinc amalgamation is also commonly done with mercuric chloride.
Mercuric chloride is used in a umpolung reaction to remove dithiane groups attached to a carbonyl. Also, the use of mercuric chloride as a stabilizing agent can benefit chemicals and analytical samples.
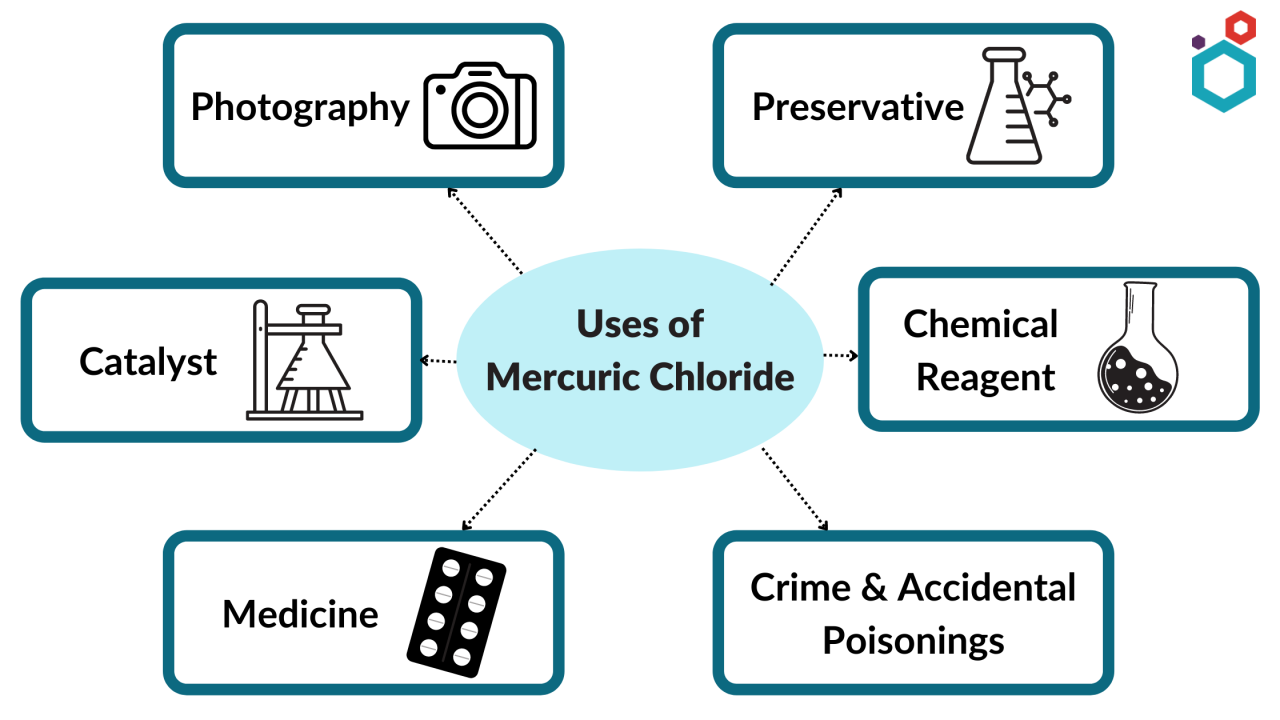
Used in photography
Mercury(II) chloride has been used as a photographic intensifier in the 1800s collodion process to produce positive images. When applied to a negative, mercury(II) chloride thickens the image, increasing the opacity of the shadows and giving the impression of a positive image.
Used in preservation
In the 19th and 20th centuries, objects were dipped in or painted with a “mercuric solution” to preserve anthropological and biological specimens. This was done to protect the specimens from moths, mites, and mould. By sprinkling crystalline mercuric chloride on objects in drawers, they were shielded. It plays a minor role in tanning and was once used to kyanize wood (soaking in mercuric chloride). Mercuric chloride was one of three chemicals used to treat railroad tie wood in Europe and the United States between 1830 and 1856. The process was largely abandoned because mercuric chloride was water-soluble and ineffective over time, as well as highly poisonous. Alternative treatment methods like copper sulfate, zinc chloride, and eventually creosote were also found to be less toxic.
Used in medicine
Mercuric chloride was a common over-the-counter disinfectant in the early twentieth century, and it was recommended for everything from fighting measles germs to protecting fur coats. In 1913, Carlin Philips of New York wrote, “It is very popular and effective household antiseptics, but it is so corrosive and poisonous that it should only be available by prescription.” A group of medical practitioners in Chicago made the same demand later that month. The product was also frequently used as a means of suicide.
Mercuric chloride was widely used to treat syphilis before antibiotics were developed. It could be inhaled, swallowed, injected, or applied topically. Both mercuric-chloride syphilis treatment and poisoning during treatment were so common that the latter’s symptoms were frequently misdiagnosed as syphilis symptoms.
Used in crime and accidental poisonings
The story of Antoine François Desrues, who used “corrosive sublimate” to murder a noblewoman, Madame de Lamotte, is told in volume V of Alexandre Dumas‘ Celebrated Crimes. After mistaking bichloride of mercury tablets for lithium citrate tablets, Richard Tilghman died in New York in 1906. In one well-publicized case in 1920, the death of 25-year-old American silent-film star Olive Thomas was attributed to “mercury bichloride.”
While on vacation in France and staying at the Hôtel Ritz in Paris, she inadvertently (or perhaps intentionally) ingested the compound, which had been prescribed to her husband Jack Pickford in liquid topical form to treat his “chronic syphilis.” Thomas died five days later.
Side effects of Mercuric Chloride
- Mercuric chloride is extremely toxic in both the short and long term. It is toxic due to its mercury content and corrosiveness, which can result in serious internal damage such as stomach ulcers, mouth ulcers, and throat ulcers, as well as corrosive intestinal damage.
- It builds up in the kidneys, causing severe chemical burns and potentially leading to acute renal failure.
- Acute mercury chloride poisoning can cause a burning mouth and throat, stomach pain, abdominal discomfort, drowsiness, hematemesis, corrosive bronchitis, severe gastrointestinal inflammation, and renal failure.
- Insomnia, slowed reflexes, excessive salivation, gum bleeding, malaise, tremors, and tooth problems are all symptoms of chronic exposure to mercury.
- Acute exposure to high mercury chloride concentrations can cause death within 24 hours due to acute renal failure or gastrointestinal disability. Acute exposure victims in other cases took up to two weeks to die.
FAQs
Q. Is Mercuric Chloride toxic?
Mercuric chloride is highly toxic both directly and as well as a cumulative toxin. Its toxic nature is not only due to the content of mercury but also due to the corrosive properties of this compound, which can cause significant internal damage, including stomach, mouth and throat ulcers, and corrosive bowel damage if ingested.
Q. What is Mercuric Chloride used for?
Mercuric Chloride is an odourless, white crystal or powder. It is used in preserving wood, photography, embalming, fabric printing and analytical chemistry, and as a disinfectant, fungicide and insecticide.
Q. Where to buy Mercuric Chloride?
Macsen Laboratories is the leading manufacturer and supplier of Mercuric Chloride with ISO certification.
Q. Why Mercuric Chloride is called Corrosive Sublimate?
Mercuric chloride does not exist as a salt composed of discrete ions, but rather is composed of linear triatomic molecules, hence it has the tendency to sublime.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of Mercuric Chloride. We do not supply or promote our Mercuric Chloride product for the applications which are covered by valid patents and which are not approved by the FDA.
Macsen Labs is a manufacturer and supplier of several grades of Mercuric Chloride such as:-

