Table of Contents
What are Mercury Salts ?
Mercury salts refer to chemical compounds that contain mercury combined with one or more other elements or groups, known as anions. These compounds are derived from the element mercury (Hg) and can exhibit a wide range of chemical and physical properties.
Mercury salts can be classified into two main categories based on the oxidation state of mercury:
Mercuric salts: These salts contain mercury in the +2 oxidation state (Hg2+). Examples of mercuric salts include mercuric chloride (HgCl2), mercuric sulfate (HgSO4), and mercuric nitrate (Hg(NO3)2). They are typically more stable and less toxic than mercurous salts.
Mercurous salts: These salts contain mercury in the +1 oxidation state (Hg+). Examples of mercurous salts include mercurous chloride (Hg2Cl2) and mercurous nitrate (Hg2(NO3)2). Mercurous salts are generally less stable and more toxic compared to mercuric salts.
Examples of Mercury Salts
Mercuric Acetate
Mercuric acetate is a chemical compound with the formula Hg(CH3COO)2. It is also known as mercury(II) acetate. The compound is generally formed by the reaction between mercury(II) oxide and acetic acid. It is a yellowish-white crystalline solid with a strong acetic acid odor and is soluble in water and organic solvents such as ethanol. It is a toxic substance and should be handled with caution.
Mercuric acetate is mainly used in the synthesis of organomercury compounds. It can act as an oxidizing agent and is employed in various chemical reactions, including the conversion of alkenes to epoxides, oxymercuration-demercuration reaction and desulfurization procedures.

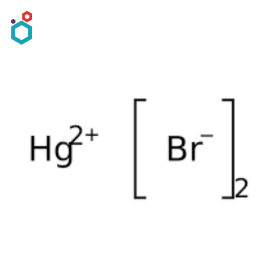
Mercuric Bromide
Mercuric bromide, also known as mercury(II) bromide, is a chemical compound with the formula HgBr2. It is a coordination compound and can be formed by the reaction of metallic mercury with bromine. It is a white crystalline solid that is slightly soluble in water and more soluble in ethanol. It has a high melting point of 237 °C.
Mercuric Bromide is mainly used as a laboratory reagent such as in the Koenigs–Knorr reaction. Additionally, it has potential use as a promoter in the glycosylation reaction of alcohols.
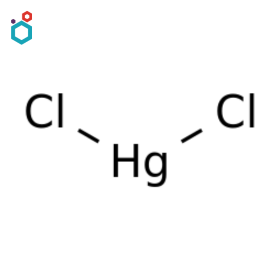
Mercuric Chloride
Mercuric Chloride, or Mercury (II) Chloride, Mercury bichloride or Dichloromercury, is a crystalline white solid with the chemical formula HgCl2. It was also known as “sulema” or “corrosive sublimate”. The compound is formed by the reaction between mercury and chlorine or by the addition of hydrochloric acid to a hot, concentrated solution of mercury(I) compounds.
Mercuric Chloride is used as a catalyst and chemical reagent in various reactions, such as inorganic synthesis and in the preparation of other mercury compounds. It is also used in some electrochemical processes. It has a history of being used as a disinfectant, preservative and as a photographic intensifier. Mercuric chloride is highly toxic both directly and as well as a cumulative toxin.
Resources: Mercuric Chloride | Chemical Properties, Uses and Side Effects
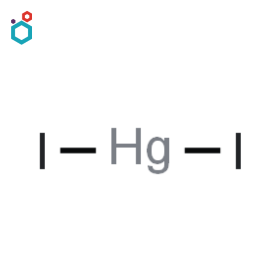
Mercuric Iodide
Mercuric iodide, also known as mercury(II) iodide, is a chemical compound with the formula HgI2, formed by the reaction between potassium iodide (aq. sol.) and mercury(II) chloride (aq. sol.). Mercuric iodide can exist in two forms: a red form and a yellow form. The red form is composed of long needle-like crystals and is more stable at higher temperatures. The yellow form consists of small plate-like crystals and is more stable at lower temperatures. The red form is the more commonly observed one. Also, It is notable for its interesting property called thermochromism. The red form is sensitive to temperature and can undergo a color change to yellow upon heating above 126 °C (400 K).
It is sparingly soluble in water. Mercuric Iodide is used for the preparation of Nessler’s reagent in laboratories. It is used in blister ointments in veterinary medicine. It is also used in some x-ray and gamma ray detection and imaging devices.
Mercuric Nitrate
Mercuric nitrate is an inorganic chemical compound with the formula Hg(NO3)2. It is a colorless to white, crystalline solid that is soluble in water, nitric acid, acetone, and ammonia. Mercury (II) Nitrate is generally synthesized by treating mercury with hot concentrated nitric acid.
When solid Mercuric nitrate is heated, it decomposes to form solid mercury(II) oxide, gaseous nitrogen dioxide, and oxygen. It has been used in various applications, including as a reagent in chemical laboratories for certain analytical procedures such as in mercuration of ketones.

Mercuric Oxide
Mercuric Oxide is an inorganic chemical compound with the formula HgO. It exists in two forms: red mercuric oxide and yellow mercuric oxide. Red mercuric oxide (also called “red precipitate” or “mercury(II) oxide”) is obtained by heating mercury metal in the presence of oxygen or by the thermal decomposition of mercury(II) nitrate. It is a red or reddish-brown crystalline powder that is insoluble in water. Yellow mercuric oxide (also called “yellow precipitate” or “mercury(I) oxide”) is formed by treating a hot solution of mercuric chloride with an alkali, such as sodium hydroxide. It is a yellow or orange powder that is slightly soluble in water.
Mercuric Oxide is used as a chemical intermediate for mercury salts, organic mercury compounds, and chlorine monoxide. It also serves as a cathode material in mercury batteries.
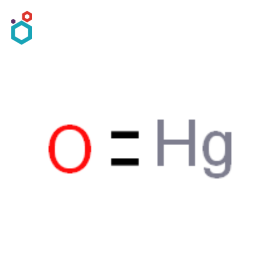
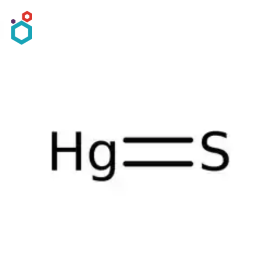
Mercuric Sulfide
Mercuric sulfide, also known as mercury(II) sulfide or cinnabar, is a chemical compound with the formula HgS. It is dimorphic with two crystal forms- Red cinnabar and Black metacinnabar. Mercuric sulfide appears as odorless red or black solid, is insoluble in water and has a high density of 8.10 g/cm3.
Historically, it has been used as a pigment for red dyes, paints, and inks due to its vibrant color. It was also used as a mercury cell in the chlor-alkali industry (Castner–Kellner process). It can also be utilized as photo-electrochemical cells because of its band gap of 2.1eV and its stability.
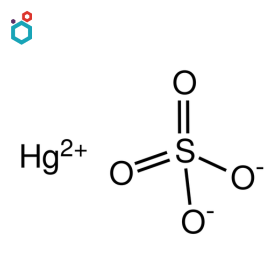
Mercuric Sulfate
Mercuric Sulfate, or Mercury (II) Sulfate, is an inorganic chemical compound with the formula HgSO4. It exists as an odourless white or colourless crystalline solid powder or granules. It is soluble in water and forms an acidic solution due to the presence of the sulfate ion.
Mercuric sulfate has been used in various applications, including as a laboratory reagent like Denigés’ reagent, as a catalyst in organic synthesis such as in the synthesis of acetaldehyde and in oxymercuration-demercuration reactions.
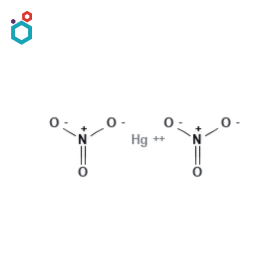
Mercurous Nitrate
Mercurous nitrate, also known as Mercury(I) nitrate or Mercuric(I) nitrate, is a chemical compound with the formula Hg2(NO3)2. It is a white, crystalline solid that is sparingly soluble in water. It usually exists in dihydrate form.
It is primarily used in certain chemical reactions and as a starting material for the synthesis of other mercury compounds. The solution of mercurous nitrate in diluted nitric acid is referred to as Millon’s reagent, and it is used as an indicator to determine the presence of tyrosine-containing proteins.
Mercurous Chloride
Mercurous chloride, also known as calomel, is a chemical compound with the formula Hg2Cl2. It is a white, crystalline odourless solid. The name “Calomel” derives from Greek kalos (beautiful) and melas (black) because it turns black on reaction with ammonia.
It has a long history of medicinal use, primarily as a purgative and a treatment for various ailments. However, its use has diminished due to concerns about the toxicity of mercury compounds. In modern times, it is mostly utilized in specialized applications, such as in electrochemistry and as a reference electrode in pH measurement.
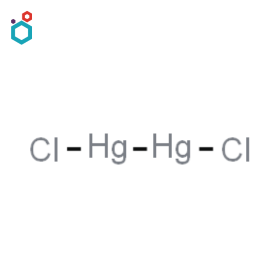
Mercuric Thiocyanate
Mercuric thiocyanate, also known as mercury(II) thiocyanate, is a chemical compound with the formula Hg(SCN)2. It is an odourless white solid crystalline powder. It is insoluble in water, but soluble in dilute hydrochloric acid, KCN, and ammonia. It is known for its unique decomposition reaction, which produces a coiling, serpent-like structure when heated. This reaction is often demonstrated as a pyrotechnic effect, creating a twisting, expanding mass that resembles a serpent or a growing tree, an effect known as the Pharaoh’s serpent.
Mercuric thiocyanate has few uses in chemical synthesis which include serving as precursor to some other mercury compounds. It is also used in analysis of chloride ions present in water by UV-visible spectroscopy.

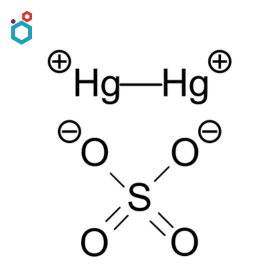
Mercurous Sulfate
Mercurous Sulfate, also known as Mercury(I) Sulfate, is a chemical compound with the formula Hg2SO4. It is a metallic salt formed by replacing both hydrogen atoms in sulfuric acid with mercury(I). It is a white, pale yellow to beige solid powder. It is insoluble in water and soluble in dilute nitric acid and hot sulfuric acid.
Mercury(I) sulphate is utilized frequently in electrochemical cells owing to its low solubility and ability to sustain large potential.
Potential hazards associated with Mercury Salts
The use of mercury compounds poses several potential hazards due to the toxic nature of mercury. Here are some of the key hazards associated with mercury compounds:
Health effects
Mercury is a highly toxic substance that can cause serious health problems in humans. Inhalation, ingestion, or skin absorption of mercury compounds can lead to a range of health effects, including damage to the nervous system, kidneys, and lungs. It can also cause developmental issues in unborn children and pose a particular risk to pregnant women.
Environmental impact
Mercury is a persistent environmental pollutant. Improper disposal or release of mercury compounds can contaminate water bodies, soil, and the air. Once released, mercury can accumulate in the food chain, leading to bioaccumulation and biomagnification in organisms, including fish and other wildlife. This poses a threat to ecosystems and can impact human health through the consumption of contaminated food.
Chemical reactivity
Some mercury compounds can be reactive and pose risks of fire, explosion, or toxic gas release when exposed to certain conditions, such as heat, light, or incompatible substances. It is essential to handle and store mercury compounds appropriately to prevent accidents and chemical reactions.
Biohazardous waste
Mercury compounds are considered hazardous waste and require special handling and disposal procedures. They should not be discarded in regular waste streams or poured down drains as they can contaminate water sources and harm the environment. Proper disposal methods, following local regulations, should be implemented to minimize the risk of mercury exposure.
FAQs
Q. What is mercury poisoning and its symptoms ?
Mercury poisoning is a form of metal toxicity caused by exposure to mercury or its compounds. Symptoms are dependent on exposure type, dose, route, and duration. These symptoms may include muscle paralysis, poor coordination, numbness in the hands and feet, skin rashes, anxiety, memory issues, difficulty in speaking, hearing or seeing, decreased intelligence, kidney problems, etc.
Q. Is mercury a metal ?
Mercury is a metal, more specifically “heavy metal”. It is one of its kind as it is the only metal that exists in a liquid state at room temperature.
Q. What color is mercury ?
Mercury has a silvery appearance. In its pure form, it has a reflective, metallic, and shiny surface, resembling the color of silver, thus is often referred to as a “silver liquid.”
Q. What is mercury metal made of ?
Mercury element is found in earth’s crust. It is found either as a native metal (rare) or in cinnabar, metacinnabar, sphalerite, corderoite, livingstonite and other minerals, with cinnabar (HgS) being the most common ore. It can also be found in hot springs or other volcanic regions
Q. What is the density of mercury ?
Mercury has a density of 13.534 g/cm3.
Q. Who discovered the mercury element ?
Mercury was discovered in Egyptian tombs, dating back to the 15th century B.C.
Q. Is mercury a liquid ?
Mercury exists in liquid state at room temperature as it has a very low melting point of −38.83 °C.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc. and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of our Mercury Salts. We do not supply or promote our Mercury Salts for any hazardous applications.
Macsen Labs is a manufacturer and supplier of a range of Inorganic Fine Chemicals and Metal Salts.