Table of Contents
What is Prussian Blue?
Prussian blue is a dark blue pigment that has been used in a variety of applications, including art, medicine, and technology. The pigment is made by reacting iron(II) salts with ferricyanide salts to create a highly insoluble blue compound known as iron hexacyanoferrate(III), Berlin blue or Brandenburg blue. It is known to contain a hydrated iron(III) hexacyanoferrate(II) anion, {FeIII[FeII(CN)6]·xH2O}−, with varying values of x up to 16, and various cations, such as K+, NH4+, or Na+ .
Prussian blue consists of a group of the two compounds, namely insoluble Prussian blue and soluble Prussian blue:
- Water-insoluble Prussian blue: Fe4[Fe(CN)6]3
- Water-soluble Prussian blue (containing alkali cations): KFe[Fe(CN)6]
The pigment was first discovered in the early 18th century by a Berlin-based color maker named Johann Jacob Diesbach, who was attempting to create a red pigment. Instead, he accidentally created a blue pigment that was more vibrant and resistant to fading than other blue pigments available at the time.
Prussian blue has been used in art to create blue hues in paintings, prints, and textiles. It has also been used in medicine to treat radiation exposure and certain types of heavy metal poisoning. In addition, it has been used in the production of blueprints, as a dye for paper, and as an indicator for the presence of certain metal ions.
| PRUSSIAN BLUE SPECIFICATIONS | |
|---|---|
| Name of Product | Prussian Blue |
| IUPAC Name | iron(2+);iron(3+);octadecacyanide |
| Synonyms | Ferric Ferrocyanide; iron(III) hexacyanoferrate(II); Ferrocin; Parisian blue; Preussischblau; Turnbulls Blau; Berliner Blau; Brandenburg blue; Berlin blue; Sarum blue; Midnight blue |
| CAS No | 14038-43-8 |
| Molecular Formula | C18Fe7N18 |
| Molecular Weight | 859.2 g/mol |
| Appearance (Form) | Solid Powder or Opaque Crystals |
| Appearance (Color) | Deep Blue to Purple |
| Solubility | Practically insoluble in water, diluted acids, and most common organic solvents |
| Pubchem CID | 2724251 |
| Pubchem SID | 475523906 |
Uses of Prussian Blue
Prussian blue has been used in a variety of applications, including:
- Art: Prussian blue is a popular pigment used in painting, printmaking, and other forms of visual art. It is valued for its deep blue color and its lightfastness, which means that it resists fading when exposed to light.
- Medicine: Prussian blue has been used as a treatment for certain types of heavy metal poisoning, including thallium and radioactive cesium and thallium isotopes. It is provided orally as an antidote and works by binding to these metals in the body and preventing their absorption.
- Imaging: Prussian blue is used as a contrast agent in medical imaging, particularly for magnetic resonance imaging (MRI). It helps to enhance the visibility of certain tissues and structures in the body.
- Analytical chemistry: Prussian blue is used as a reagent in analytical chemistry to detect the presence of certain metal ions. It is particularly useful for detecting iron, copper, and cesium ions.
- Industrial applications: Prussian blue has been used in a variety of industrial applications, such as the production of blueprints and as a dye for paper and textiles. It is also used in electroplating and as electrode materials in the production of certain types of batteries, such as lithium-ion, sodium-ion, and potassium-ion batteries for reversible alkali-ion insertion and extraction.
- It is a common histopathology stain that pathologists employ to detect the presence of iron in biopsies, such as bone marrow samples.
Prussian Blue Analogues (PBA)
Prussian blue analogues (PBA), also known as iron cyanides or ferrocyanides, are a group of compounds that are structurally related to Prussian blue. Like Prussian blue, these compounds are made by reacting iron salts with cyanide compounds. This family of materials exists in the form of AxP[R(CN)6]1−y·zH2O, where A is an insertion ion, often potassium or sodium, P and R are transition metals (R site often occupied by iron (Fe)) and y is the number of [R(CN)6] vacancies. Cyanide groups connect transition metals P and R. The P atoms are coordinated to six nitrogen atoms, and the R atoms are coordinated to six carbon atoms, thus forming a framework with large voids. PBA offers many opportunities for structural variation and hence the properties are highly tunable.
The resulting compounds are highly insoluble and often have a distinctive blue, white or green color. There are several different Prussian blue analogues, each with a slightly different chemical structure and properties. Some of the most common Prussian blue analogues include Prussian White, Turnbull’s blue, potassium ferricyanide, and potassium ferrocyanide. Each of these compounds has different uses and applications, depending on their specific properties.
Prussian White- Prussian white (PW), also referred to as Berlin White (BW) or Everett’s Salt (ES), Na2Fe[Fe(CN)6], is the totally reduced and highly sodiated form of Prussian Blue. It is made by combining a solution of iron(II) salts with a solution of potassium ferrocyanide. It has an extremely high theoretical capacity of 170 mAh g−1.
Turnbull’s blue- Turnbull’s blue is a type of Prussian blue analogue that is used in photography as a toner to create blue or brown tones in black and white photographs. It is also used in electroplating and as a catalyst in certain chemical reactions.
Potassium ferricyanide- Potassium ferricyanide is a red-colored Prussian blue analogue that is often used as an oxidizing agent in chemical reactions. It is also used in photography, as a food additive, and in certain medical applications.
Potassium ferrocyanide- Potassium ferrocyanide is a yellow-colored Prussian blue analogue that is used in the production of dyes, pigments, and inks. It is also used in certain metal plating processes and as a food additive.
Other Prussian Blue analogues (PBAs) can also be formed by using different transition metals in place of Iron (Fe), like Manganese (Mn), Calcium (Ca), Copper (Cu), Cobalt (Co), Nickel (Ni), etc. and their properties vary accordingly.
Prussian blue analogues are important compounds with a wide range of uses in industry, photography, and chemistry.
Synthesis of Prussian Blue Analogues
In general, there are three types of Prussian Blue Analogues synthesis methods, the precipitation method, the hydrothermal method, and the electrodeposition method.
Precipitation Method
This is one of the most common methods for synthesizing Prussian Blue analogues, in which a solution of metal-ion compounds (such as Fe²⁺, Co²⁺, Ni²⁺, Mn²⁺) is combined with a solution of ferrocyanide ligand (such as potassium ferrocyanide or sodium hexacyanoferrate). The reaction forms a solid precipitate, which is then washed and dried to obtain the desired Prussian Blue analogue.
Hydrothermal Method
Hydrothermal method also referred to as Solvothermal method is a popular method to prepare Prussian blue microparticles, where the decomposition of Fe(CN)64− into Fe3+/Fe2+ in acid solution is utilized to react with the residual Fe(CN)6 to form Prussian Blue. This method involves carrying out the synthesis in a closed vessel under high temperature and pressure conditions.
Electrodeposition Method
Prussian Blue analogues can also be synthesized using electrochemical methods. Electrodeposition techniques, such as cyclic voltammetry or potentiostatic methods, are employed to drive the reaction between metal ions and cyanide ions, leading to the formation of the analogue on the electrode surface.
Use of Prussian Blue Analogues in Sodium ion batteries
Sodium ion batteries are a type of rechargeable battery that use sodium ions as the charge carrier instead of the more common lithium ions. They are a promising alternative to lithium-ion batteries, as they use more abundant and less expensive materials, and are potentially safer and more environment friendly.
A sodium-ion battery typically consists of the following components-
- Cathode: The cathode is the positive electrode in the battery where sodium ions are stored during the charging process. Common cathode materials in sodium-ion batteries include sodium transition metal oxides, including Prussian Blue Analogues (PBAs). These materials allow for reversible insertion and extraction of sodium ions.
- Anode: The anode is the negative electrode in the battery which receives and releases sodium ions during the charging and discharging process. Carbon-based materials, such as hard carbon or graphite, are commonly used as anodes in sodium-ion batteries due to their ability to intercalate sodium ions.
- Electrolyte: The electrolyte is a medium that allows the movement of sodium ions between the cathode and anode while preventing direct contact between the two electrodes. Sodium-ion batteries typically use a liquid or solid-state electrolyte containing sodium salts, such as sodium hexafluorophosphate (NaPF6) or sodium bis(fluorosulfonyl)imide (NaFSI). Solid-state electrolytes, which are non-flammable and offer better safety, are actively being researched as alternatives to liquid electrolytes. The electrolyte should possess high sodium-ion conductivity and should have a suitable electrochemical window that matches the operating voltage range of the battery.
- Separator: The separator is a porous membrane that physically separates the cathode and anode while allowing the passage of sodium ions. The separator prevents short circuits and maintains the electrical isolation between the two electrodes. Polymeric membranes, such as polyethylene or polypropylene, are commonly used as separators in sodium-ion batteries.
- Current Collectors: Current collectors are conductive materials that facilitate the flow of electrons between the electrodes and the external circuit. They are typically made of metals, such as aluminum or copper, and are coated onto the cathode and anode materials.
These components work together to enable the movement of sodium ions during the charging and discharging process, which results in the storage and release of electrical energy in a sodium-ion battery.
Prussian blue analogues have been studied extensively for their potential applications in sodium ion batteries. PBA have several properties that make them attractive for use in sodium ion batteries, including high theoretical capacity, good electrochemical stability, and low cost. Some of the specific applications of Prussian blue analogues in sodium ion batteries include:
- Electrodes: Prussian blue analogues can be used as the cathode or anode in sodium ion batteries. They have been shown to exhibit good cycling stability and high capacity retention, making them promising candidates for use in commercial sodium ion batteries.
- Active material coatings: Prussian blue analogues can be used as coatings on other electrode materials to improve their performance in sodium ion batteries. For example, coating a graphite electrode with Prussian blue analogue has been shown to improve its cycling stability and increase its capacity.
- Solid electrolytes: Prussian blue analogues can also be used as solid electrolytes in sodium ion batteries. They have been shown to exhibit good ionic conductivity and could potentially be used to improve the safety and stability of sodium ion batteries. They show great promise for use in sodium ion batteries and are the subject of ongoing research and development efforts.
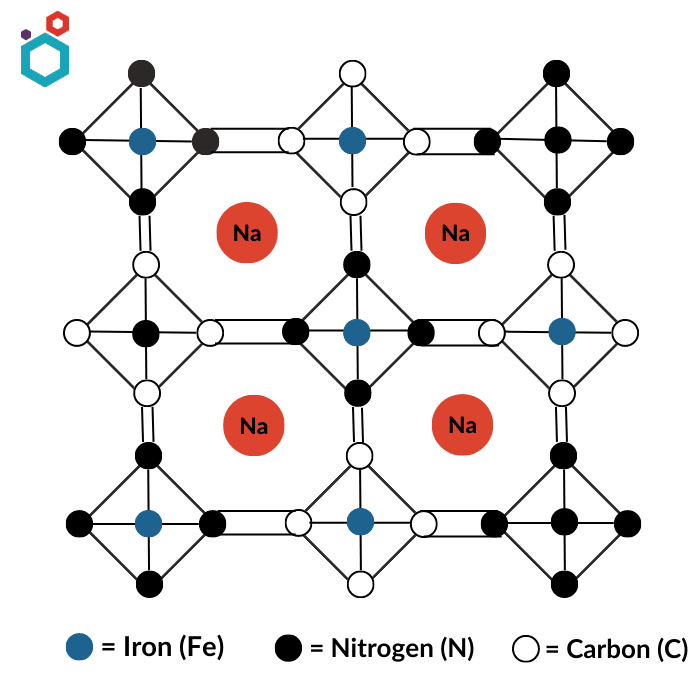
Use of Prussian White in making Sodium-ion Battery Cathodes
- Prussian White has a crystal structure that is composed of a three-dimensional network of iron(II) and iron(III) atoms, which creates sites where sodium ions can be stored through a process known as intercalation. The intercalation of sodium ions into the crystal structure of Prussian White creates a flow of electrons between the cathode and the battery terminals, which generates an electric current that can power devices.
- In addition to its ability to store sodium ions, Prussian White is also stable, safe, and low cost, making it an attractive option for use in sodium-ion battery cathodes.
- Furthermore, it has been shown to have good cycling stability and high capacity retention (theoretical capacity of 170 mAh g−1), which are important properties for battery performance over the long term.
How do prussian blue analogues work in sodium ion batteries ?

Prussian blue analogues, such as iron hexacyanoferrate (FeHCF), can be used as cathode materials in sodium ion batteries.
During the initial charging, electrical energy is employed to induce electron migration from the positive electrode to the negative electrode via an external circuit. In order to maintain charge neutrality, the positive electrode simultaneously releases sodium ions into the electrolyte. The sodium ions from the electrolyte are inserted into the structure of the FeHCF cathode, causing it to undergo a reversible electrochemical reaction. Specifically, the sodium ions react with the iron centers in the FeHCF structure, forming a reduced form of the compound known as Prussian white.
More and more sodium ions are transferred from the cathode to the anode via adsorption and intercalation processes as the battery continues to be charged.
During discharge, the stored sodium ions are released from the anode and travel through the electrolyte to the cathode, where they react with electrons to form sodium metal or another sodium compound. This process generates an electric current that can be used to power a device.
The use of Prussian blue analogues as cathode materials in sodium ion batteries has several advantages, including their low cost, high stability, and high specific capacity (i.e., the amount of charge that can be stored per unit mass or volume of the material). However, these materials also have some limitations, including their relatively low energy density (i.e., the amount of energy that can be stored per unit mass or volume of the material) and their tendency to undergo structural changes during cycling, which can lead to a loss of capacity over time. Ongoing research is focused on addressing these limitations and improving the performance of Prussian blue analogues and other cathode materials for use in sodium ion batteries.
Efficiency of PBA in sodium-ion batteries
The efficiency of different types of Prussian blue analogues for use in sodium ion batteries can vary depending on several factors, including the specific composition and structure of the material, the synthesis method used to prepare it, and the conditions under which it is used in the battery.
For instance, the rhombohedral structure of Prussian Blue is highly advantageous for its efficacy. Greater surface area, lower particle size and moisture content are some of other the factors affecting the efficiency of PBAs. The presence of even minute amounts of elemental impurities, which may be transferred over during the synthesis or from other processes, can have a significant impact on the battery’s performance, cycling stability, charge-discharge rate, and, ultimately, its efficacy.
Generally speaking, Prussian blue analogues such as iron hexacyanoferrate (FeHCF) and copper hexacyanoferrate (CuHCF) have shown promise for use as cathode materials in sodium ion batteries, with high specific capacities and good cycling stability. Other Prussian blue analogues, such as nickel hexacyanoferrate (NiHCF) and cobalt hexacyanoferrate (CoHCF), have also been investigated for their potential use in sodium ion batteries, although their performance may be more limited due to issues such as poor cycling stability or lower specific capacity. For non-aqueous Prussian Blue batteries, water-free PBAs are preferable.
Research in this area is ongoing, with efforts focused on optimizing the performance of Prussian blue analogues and developing new materials with even better properties for use in sodium ion batteries. Ultimately, the efficiency of each type of Prussian blue analogue will depend on a range of factors, and will need to be evaluated on a case-by-case basis through rigorous testing and analysis.
Macsen is a manufacturer and supplier of battery grade cathode materials-
