Home » Blog » Selenious Acid »
Table of Contents
What is Selenious Acid?
The Selenious acid, a selenium compound, is an acid form of Sodium Selenite. One of the major use of SA is in protecting and changing the colour of steel. In medicine, selenious acid is used as a source of selenium. Selenium is essential as it helps the body in making proteins that are called antioxidant enzymes which are instrumental in preventing cell damage.
Note for the reader – In a few instances, this article refers to Selenious Acid as SA.
Structure
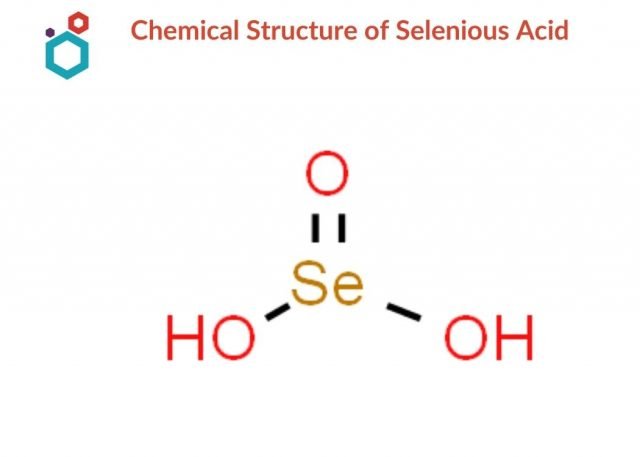
Chemical Properties
The chemical properties are mentioned in the table below-
| Property | Description |
|---|---|
| Chemical Name | Selenium dioxide |
| Synonyms | Selenige Säure, Selenous acid, Ácido selenioso, Acido selenio, Acide sélénieux |
| Appearance | Colourless, deliquescent crystal |
| Type | Small Molecule |
| Molecular Formula | H2O3Se |
| CAS No. | 7783-00-8 |
| Molecular Weight | 128.97 g/mol |
| Toxicity (If swallowed) | Acute |
| Toxicity(If inhaled) | Acute |
| Oxidation State | Strong Oxidizer |
| Stability | Stable |
| Flammability | Non-combustible |
| Heat of combustion | It emits toxic fumes of selenium |
| pH value | Approximately 5.5 (Not Confirmed) |
| Acidity or basicity | Acidic |
| Radioactivity | Radioactive |
| Solubility | Very soluble in water & ethanol and insoluble in ammonia |
| Pubchem CID | 1091 |
| Pubchem SID | 462770287 |
Other Properties
- Strongly Acidic since it’s from the sulfur family
- An amorphous non-crystalline red powdery form of selenium results when a solution of SA (H2SeO3) is treated with sulfur dioxide(so2).
Mechanism of action
Selenious acid most likely has the same mechanism of action as selenium. Selenium has shown results in cancer treatment for certain cancers, heart and vascular diseases and as an immune system stimulator.
One of the most crucial actions that selenium performs in the body is the oxidation of free sulfhydryl groups. Free sulfhydryl (-SF) groups on the surface of the cell membranes of tumour cells contribute to the rampant division of cells. Selenium being an antioxidant can oxidize these sulfhydryl groups to disulfides (S-S) and thus halt the activity of cancerous cells. However, the exact mechanism of action of selenium concerning cancer is still unknown and requires more in-depth research.
Uses of Selenious Acid
It has both medical and non-medical applications. A few of them are below-
- SA is used in solar panels. It is also used in light sensors, laser printers and photocopiers.
- SA is also used in the cold-bluing process to change the colour of steel from silver-grey
- SA injection is prescribed for total parenteral nutrition(TPN), for maintaining plasma selenium levels and also to maintain endogenous stores to prevent deficiency.
- It is also used in chemical darkening and patination of copper, brass and bronze.
- SA is used as an oxidizing agent in the synthesis of 1,2-dicarbonyl compounds.
- Selenium Sulfide, another selenium compound, is also used as an ingredient in anti-dandruff shampoos.
- SA is used as an ingredient in Mecke Reagent which is used for drug testing.
- Isotope is used in labelling radiopharmaceuticals
Side Effects
- It is highly toxic orally. SA and its salts can produce acute poisonings and are capable of penetrating the skin. It can burn the skin and can cause irritations.
- Breathing SA can irritate the nose, throat and lungs.
- Exposure can also lead to headache, nausea, vomiting, abdominal pain, pallor and fatigue.
- It can also escalate problems of depression and anxiety.
- It can damage the nervous system and can also affect the liver and kidneys.
Precautions
The biological role of Selenium is as an essential trace element for some species including humans. That’s why selenium is very important for humans to consume. Every cell in our body contains at least 14 milligrams of Selenium which is more than a million selenium atoms per cell. Too much Selenium is toxic. A great amount of selenium can be carcinogenic and teratogenic. It disturbs the development of an embryo or a fetus.
You should avoid heating It. When it is heated it emits toxic fumes of selenium.
Also, you should avoid inhalation of SA, and also avoid making skin contact. The Liquid that contains selenium inorganic compounds should be kept absorbed in some dry sand, vermiculite, earth, or any similar material.
FAQs
Q. Is Selenious acid toxic?
Selenious acid is highly toxic if it is taken in excessive quantity, just like any Selenium compound. As a dietary source in proper amounts, it is however approved. The symptoms of overdosage of SA include – Cerebral oedema, death, garlic or sour breath odour, weak nails, vomiting, gastrointestinal disturbance, hypersalivation, hair loss, and mental depression.
Q. Is Selenious acid strong?
Selenious acid (H2SeO3) is similar to sulfuric acid (H2SO4), which is strong and it is extremely soluble in water. When SA is heated it gets decomposed to toxic and volatile selenium dioxide. It also behaves as an oxidizing agent.
Q. What is the formula of Selenious acid?
The formula of Selenious acid is- H2SeO3
Q. Is Selenium the same as Selenious acid?
The acid form of sodium selenite is called Selenious acid. Sodium selenite is a form of selenium. While Selenium is an essential trace element and also an antioxidant. Selenium is a semi-metal that has the properties of silicon and arsenic. Thus it demonstrates both the properties of a metal and a non-metal depending on how it is doped, altered, and combined with other elements in the real world.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of SA. We do not supply or promote our SA product for the applications which are covered by valid patents and which are not approved by the FDA.
Macsen Labs is a manufacturer and supplier of high-quality Selenious Acid.
Other articles on Selenious Acid

