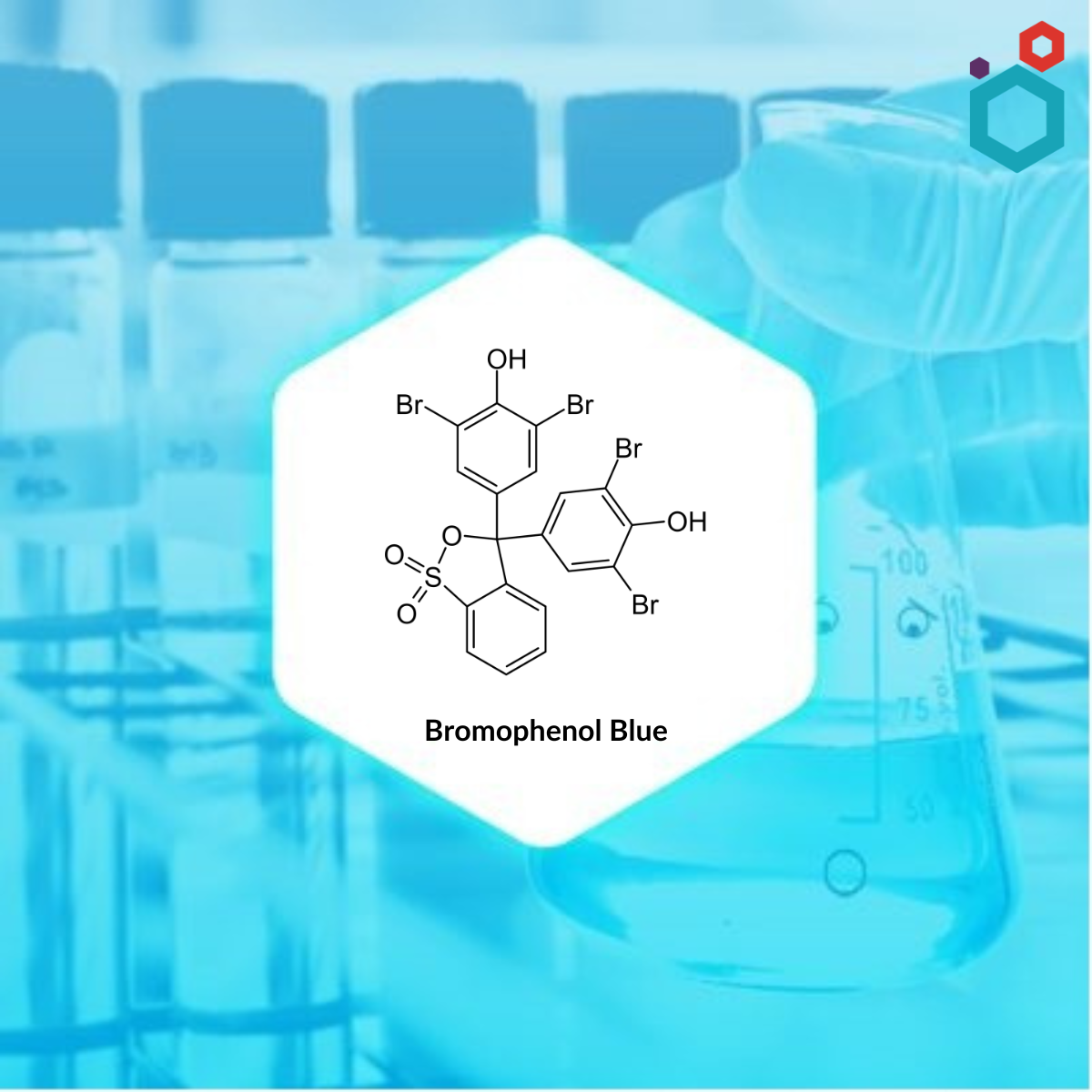
What is Bromophenol Blue?
Bromophenol Blue is an organobromide compound and a member of phenols. It is a pH indicator dye commonly used in laboratory settings. It changes color depending on the acidity or alkalinity of a solution, shifting from yellow (acidic) to blue (alkaline) as the pH increases. It’s often employed in various chemical and biological experiments to monitor pH changes. It shows maximum absorbance at 592 nm.
| PRODUCT SPECIFICATIONS | |
|---|---|
| Name of Product | Bromophenol Blue |
| IUPAC Name | 2,6-dibromo-4-[3-(3,5-dibromo-4-hydroxyphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-3-yl]phenol |
| Synonyms | Albutest; Tetrabromphenol Blue; 3,3',5,5'-Tetrabromophenolsulfonphthalein; 3′,3″,5′,5″-tetrabromophenolsulfonphthalein; BPB; Bromphenol Blue Sultone Form |
| CAS No | 115-39-9 |
| Molecular Formula | C19H10Br4O5S |
| Molecular Weight | 670.0 g/mol |
| Pubchem CID | 8272 |
CHEMICAL PROPERTIES
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | Appearance (form) | Crystalline Solid Powder |
| 2 | Appearance (color) | Dark Bluish-Black |
| 3 | Odor | Odorless |
| 4 | Solubility | Freely soluble in NaOH solutions Soluble in Methanol, Ethanol and Benzene Soluble in Water (3 mg/mL) |
| 5 | Melting Point | 273℃ |
| 6 | Density | 2.2 g/cm3 |
| 7 | pKa | 3.85 |
How does bromophenol blue indicator work?
Bromophenol Blue works as a pH indicator by changing color in response to pH variations. It’s typically yellow in acidic conditions and transitions to blue as the solution becomes more alkaline. This color shift occurs due to changes in the electronic structure of the molecule as it donates or accepts protons (H⁺ ions), making it a useful tool for tracking pH changes in various laboratory applications.
Uses
Some of the common uses of Bromophenol Blue are as follows-
- pH Indicator: It serves as a visual indicator of the pH level in solutions, transitioning from yellow (acidic) to blue (alkaline) with pH changes.
- Electrophoresis: It is used in gel electrophoresis to monitor the migration of DNA, RNA, or proteins, helping scientists analyze molecular size and charge.
- Protein Assays: In biochemistry, it aids in quantifying proteins and peptides, as they change color in response to pH shifts during assays.
- Microbiology: It’s used in microbiology to differentiate between lactose-fermenting and non-fermenting bacteria based on pH changes in growth media.
- Quality Control: Bromophenol Blue is employed in various industries for quality control and monitoring pH of products.
FAQs
Q. How to make Bromophenol Blue indicator solution?
Bromophenol Blue indicator solution can be prepared by dissolving 0.1 g of Bromophenol Blue with gentle heating in 1.5 ml of 0.1 M NaOH and 20 ml of ethanol (95%) and add sufficient water to produce 100 ml.
Q. Is Bromophenol Blue toxic?
Bromophenol Blue is considered mildly risky when it comes to skin contact (causing irritation), eye contact (causing irritation), ingestion, or inhalation.
Buy high purity Bromophenol Blue from the leading manufacturer with ISO certification. For buying, send us an enquiry-
