Table of Contents
What is Mercurochrome?
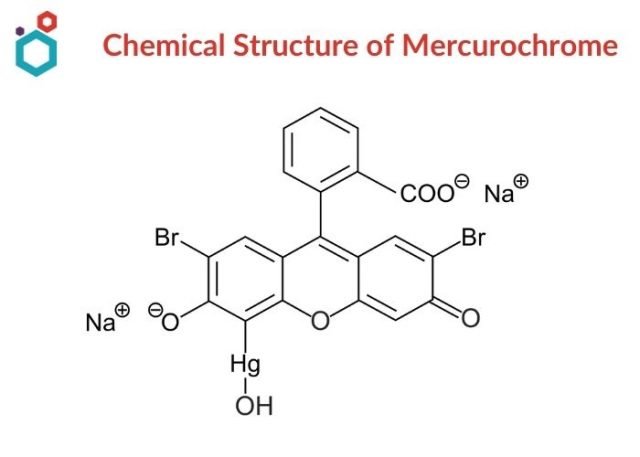
Mercurochrome was one of the first antiseptics containing mercury, a chemical element that disinfects by disrupting a microorganism’s metabolism. Mercurochrome (Merbromin, disodium salt of 2,7-dibromo-4-hydroxymercurifluorescein) is the oldest organic mercurial antiseptic in use, but it is only bacteriostatic. In its name, the suffix “mercuro” is for its mercury content and “chrome” is for its colouring properties. It’s absorbed through burned or otherwise absorptive surfaces and can induce severe and deadly intoxication.
Mercurochrome (also known as Merbromin, Mercurichrome, Mercurochromee, Mercurocol, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept, and Cinfacromin) is an organomercuric disodium salt compound that is used as a topical antiseptic and a biological dye for minor cuts and scrapes. It is widely available in most countries, but due to its mercury content, it is no longer sold in Iran, Germany, Switzerland, Brazil, France, or the United States.
Absorption of organic mercury compounds which are used as antiseptics can cause kidney damage in young children; in one case, regular administration of mercurochrome to an omphalocele probably ended in anuria, respiratory arrest, and death. Mercurochrome and thiomersal are not recommended for the treatment of big omphaloceles.
Chemical properties of Mercurochrome
- Mercurochrome is an organic sodium salt i.e., disodium;[2,7-dibromo-9-(2-carboxylatophenyl)-3-oxido-6-oxoxanthen-4-yl]mercury;hydrate.
- It has a chemical formula of C20H8Br2HgNa2O6 and a molecular weight of 751 daltons.
- It is freely soluble in water giving a carmine-red solution and is practically insoluble in alcohol, acetone, chloroform, and ether.
- Mercurochrome, also known as Merbromin, was the first of a series of antiseptics containing mercury, a chemical element that disinfects by disrupting a microorganism’s metabolism.
- It is an antiseptic that is used to keep tiny cuts and abrasions from becoming infected but a weaker one and has been replaced in medicine by more effective members of the series, such as nitromersol (Metaphen) and thimerosal (Merthiolate), as well as antibiotics.
- It causes tissue to turn a vivid red with a yellow-green light around it.
A list of related compounds of Mercurochrome
| Compound | Chemical Formula |
|---|---|
| [2,7-dibromo-9-(2-carboxylatophenyl)-6-oxido-3-oxo-3H-xanthen-5-yl](hydroxy)mercury | C20H9Br2HgO2 |
| [2,7-Dibromo-9-(2-carboxyphenyl)-3-hydroxy-6-oxoxanthen-4-yl]mercury | C20H9Br2HgO5 |
| Merbromina | C20H9Br2HgNa2O6 |
| Eosin | C20H6Br4Na2O5 |
| 2-(2,4,5,7-Tetrabromo-6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic acid | C20H8Br4O5 |
Refer here for more details.
Synthesis
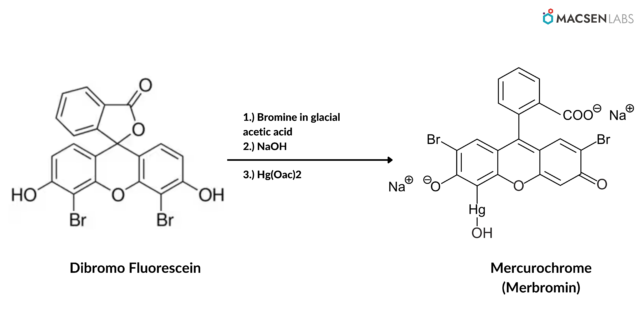
Dibromo fluorescein is combined with mercuric acetate and sodium hydroxide to make mercurochrome. It can also be synthesized by using mercuric acetate in combination with Dibromofluorescein sodium. One of its most notable chemical properties is its anionic character.
Because of its anionic nature, mercurochrome cannot be mixed with the majority of alkaloid salts and most local anaesthetics because they are acidic in nature, resulting in dangerous exothermic reactions that release toxic gases or worsen a non-fatal injury. As a result, a mixture of ethyl alcohol and aqueous water is available on the market.
Ingredients in Mercurochrome
- Dibromofluorescein
- Mercuric acetate
- Sodium Hydroxide
Recent Patents
| Patent Name | Date |
|---|---|
| Antimicrobial handle cover | 01-06-2021 |
| Medical cotton swab medicine dipping device for surgery and using method | 13-01-2021 |
| Medicine dipping mechanism, medical cotton swab medicine dipping device for surgery and using method | 13-01-2021 |
| Device for disinfection and temporary storage | 13-08-2020 |
Refer here for more details.
Uses of Mercurochrome
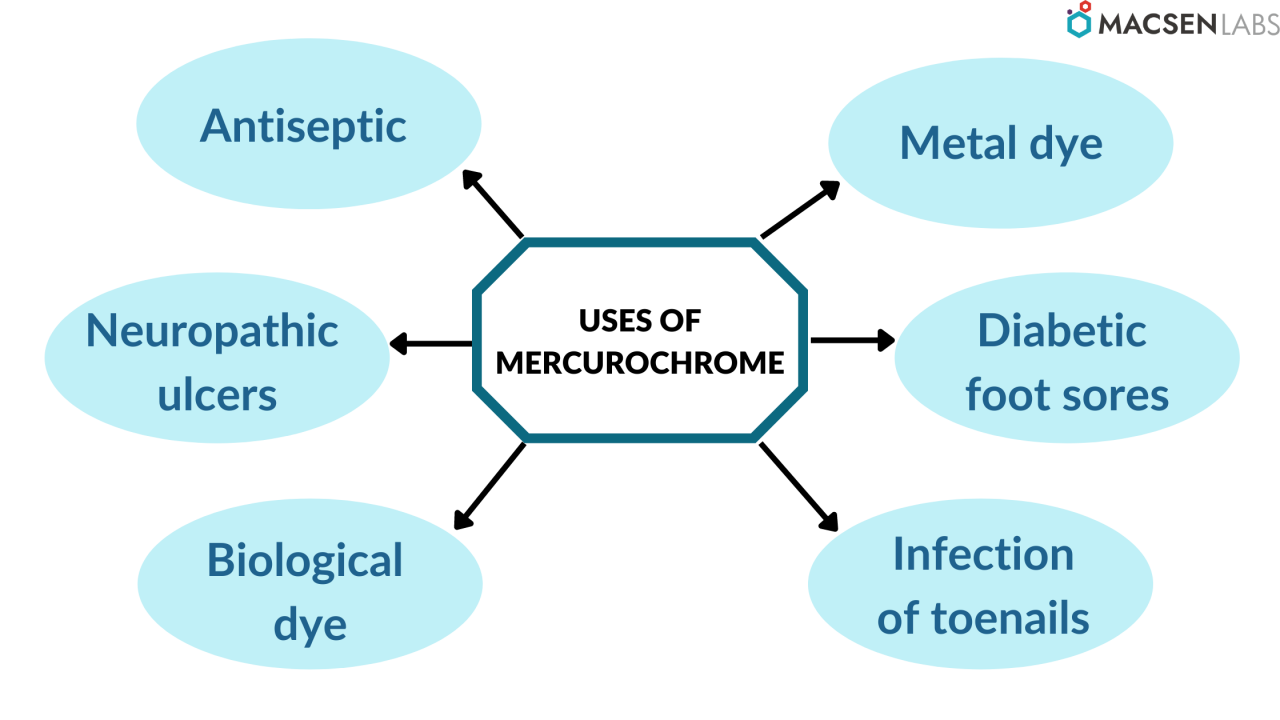
- Mercurochrome solution is a widely accepted and well-known antiseptic for the treatment of burns, minor wounds, and scratches.
- It is used in the antisepsis of wounds and the antisepsis of the umbilical cord with inhibited scar formation, such as neuropathic ulcers and diabetic foot sores, due to similar properties.
- It is also used for the escharotic treatment of big omphaloceles in neonates.
- Because of its persistence and lethality to bacteria, mercurochrome solution is also used to treat infections of toenails.
- It is also used in scientific experimentation for staining biological samples. It’s a biological dye that’s used to mark the tissue’s edges.
- Mercurochrome solution can be used as a metal dye in industrial dye penetrant inspection to detect metal fractures.
Mercurochrome poisoning (Side effects)
- When mercurochrome is swallowed, it causes mercurochrome poisoning as it is a chemical compound made up of mercury and bromine which are poisonous substances and can be harmful.
- It can be absorbed topically via the omphalocele sac, resulting in mercury intoxication and death. Silver sulfadiazine and gentian violet are two alternatives to mercurochrome that are not harmful.
- Small amounts of elemental mercury vapor escape from dental amalgam restorations into the mouth’s oral cavity. The discharged mercury can be picked up by the saliva and then dispersed throughout the body to various organs and compartments. Daily mercury uptake rates from the amalgam are predicted to range between 2 and 25 g Hg/24 h, with the ‘worst case’ individual receiving 70 g Hg/24 h.
- Mercury is a known neurotoxicant, and in certain persons, mercury off-gassing can produce dementia-like symptoms.
- Animals ingesting this substance, whether accidentally or intentionally, can have local effects ranging from minor discomfort to complete tissue necrosis in the gastrointestinal tract, as well as systemic consequences.
Comparison with other compounds
Mercurochrome vs Merthiolate
- Merthiolate is the trade name for thiomersal. Mercurochrome is the trade name for Merbromin.
- Merthiolate is a white/slightly yellow powder whereas Mercurochrome is a dark red liquid.
- Merthiolate can be used as an antiseptic and antifungal agent. On the other hand, Mercurochrome is used as an antiseptic and biological dye.
Mercurochrome vs Iodine
- Iodine is used as a tincture, comprising elemental iodine and/or sodium iodide in alcohol, whereas Mercurochrome is a compound composed of mercury and bromine.
- Iodine is a non-metal and mercury (in mercurochrome) is a metal element that is liquid at room temperature.
- Iodine tincture is more efficacious in killing bacteria & viruses as compared to Mercurochrome.
- Both are used as topical antiseptics.
Mercurochrome vs Betadine
- Mercurochrome is an organomercuric disodium salt compound. Betadine is a brand name for povidone-iodine, an iodine-based antiseptic.
- Mercurochrome works as an antiseptic due to the antimicrobial properties of mercury whereas Betadine releases iodine slowly, which kills microorganisms by disrupting their protein and nucleic acid structure.
- Mercurochrome poses serious safety concerns over potential mercury poisoning , especially with repeated use or in certain vulnerable populations, such as pregnant women and small children. Betadine, on the other hand, is widely used and considered safe for the antiseptic treatment of wounds, skin disinfection before surgery, and for the treatment of common skin infections.
FAQs
Q. Why is Mercurochrome banned?
The U.S. Food and Drug Administration (FDA) banned mercurochrome as an over-the-counter antiseptic in 1998 due to its inefficacy in killing micro-organisms, its staining property and the fear of mercury toxicity from mercurochrome being absorbed through the skin. However, it is still widely used in some countries outside the U.S.
Q. Is Mercurochrome safe to use?
Mercurochrome is a compound composed of mercury and bromine. It is dangerous if swallowed.
Q. What happens when you drink Mercurochrome?
How well someone does is determined by the amount of mercurochrome they ingested and how quickly they were treated. May be fatal if swallowed. The sooner medical assistance is provided, the better the chances of recovery are.
Q. Is mercurochrome good for wounds?
An aqueous solution of mercurochrome exhibits antiseptic properties and is used to prevent infection in minor wounds. The mercury component of mercurochrome is useful as it acts as a disinfectant, stopping the bacteria from reproducing and spreading.
Q. Is mercurochrome anti-fungal?
In a study conducted, it was found that mercurochrome is found to be efficacious in terms of healing, relief from symptoms of the disease and production of negative fungal cultures. Also, mercurochrome is recommended as a safe and economical drug for the topical treatment of otomycosis in developing countries like Nigeria.
Q. Is mercurochrome available in Canada?
In Canada, Jean Coutu Group markets a chlorhexidine solution under the name Mercurochrome.
Q. Does mercurochrome stains the skin?
When applied to a wound, it leaves a unique carmine-red stain on the surrounding tissue.
Q. How do you get Mercurochrome off your skin?
In a solution of 1-quart warm water, 1/2 teaspoon light-duty liquid detergent, and 1 tablespoon ammonia, soak for 30 minutes then rinse.
Q. Where can you buy Mercurochrome?
Macsen Labs is a GMP & ISO Certified Manufacturer and supplier of high-quality Mercurochrome.
Q. Can you use expired mercurochrome?
Expired mercurochrome should not be used and should be properly discarded.
Q. How do you use mercurochrome?
Mercurochrome or Merbromin is used as a topical antiseptic to treat minor cuts and scrapes.
Q. Can you still buy or use mercurochrome?
It had been widely used as a household antiseptic/antibacterial ointment, especially for children, because it did not hurt or irritate the skin upon application; however, it is no longer available in the United States, in part due to its mercury concentration.
Disclaimer-
The information provided here is based on general knowledge, articles, research publications etc and we do not claim the authenticity of any of the information provided above. We do not claim or suggest/advise any medical, therapeutic, health or nutritional benefits of Mercurochrome. We do not supply or promote our Mercurochrome product for the applications which are covered by valid patents and which are not approved by the FDA.
Macsen Labs is a manufacturer and supplier of several grades of Mercurochrome and Merbromin such as:-

