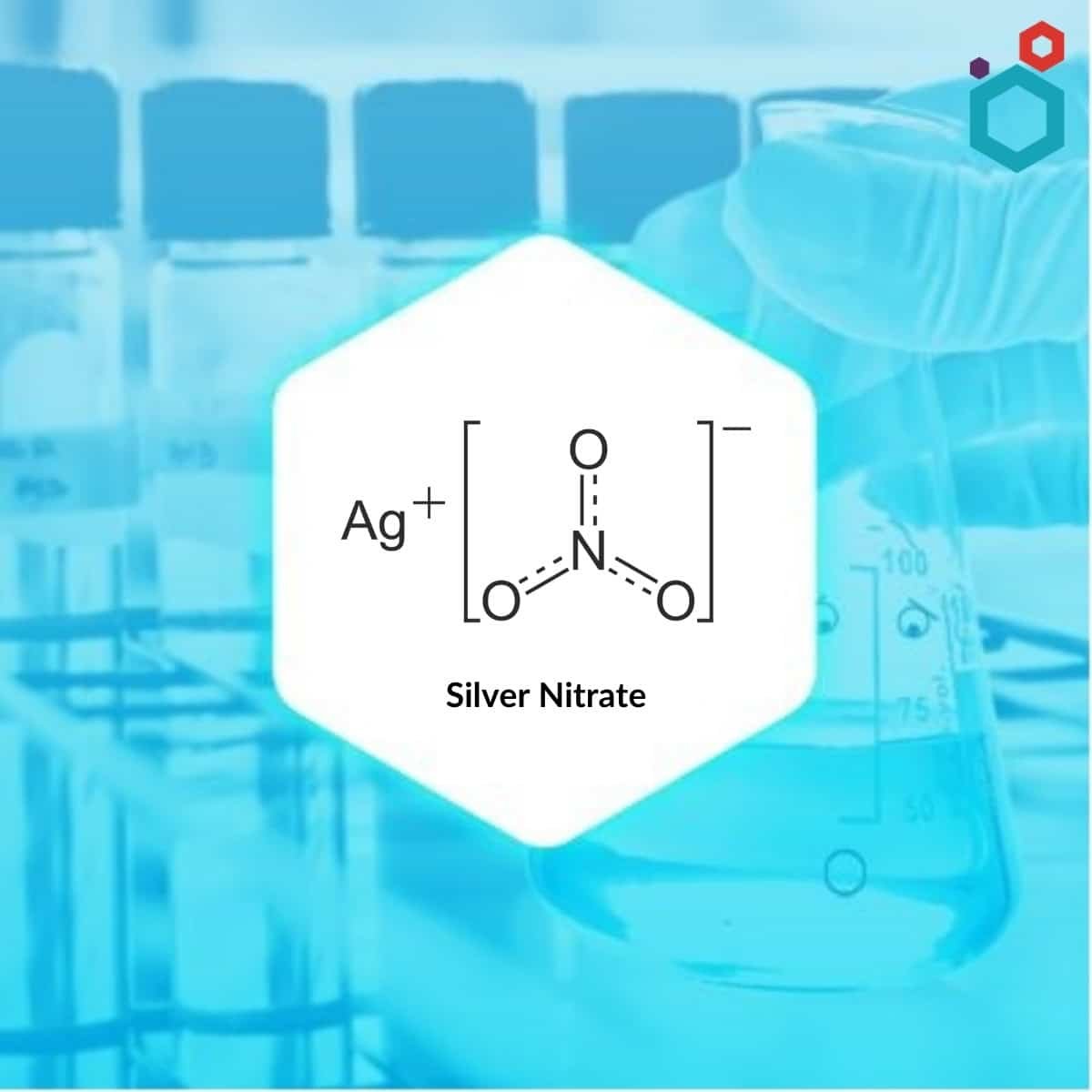
What is Silver Nitrate
Silver nitrate also called Lunar caustic, is an inorganic compound with the chemical formula AgNO3 which occurs as an odourless, colourless or white crystalline solid with an orthorhombic structure. The chemical reaction between Silver (Ag) and Nitric Acid (HNO3) results in the formation of Silver Nitrate. It has antiseptic properties. It has a wide range of applications in many fields such as biology, chemical synthesis, and medicine.
Macsen Labs is a manufacturer and supplier of several grades of Silver Nitrate such as – Silver Nitrate IP/BP and Silver Nitrate USP.
| PRODUCT SPECIFICATIONS | |
|---|---|
| Name of Product | Silver Nitrate |
| IUPAC Name | silver;nitrate |
| CAS No | 7761-88-8 |
| Synonyms | Lunar caustic; Silver (1+) nitrate; Nitric acid, silver (1+) salt; nitrato de prata; nitrato d'argento; nitrato de plata; nitrate d'argent |
| Molecular Formula | AgNO3 |
| Molar Mass | 169.873 g/mol |
| Pubchem CID | 24470 |
| Pubchem SID | 462768908 |
| GMP | ✓ |
| DMF | ✓ |
SPECIFICATIONS
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | APPEARANCE | White or almost white, crystalline powder or transparent, colorless crystals. |
| 2 | SOLUBILITY | Very soluble in water, soluble in ethanol (96%) . |
| 3 | IDENTIFICATION | A. 10mg gives the reaction of nitrates. B. 10mg gives the reaction of silver. |
| 4 | APPEARANCE OF SOLUTION | Solution is clear and colorless. |
| 5 | Foreign salts | Maximum 0.3% |
| 6 | Acidity or alkalinity | Must pass test |
| 7 | Aluminum, lead, copper and bismuth | Must pass test |
| 8 | Assay | 99.0 % to 100.5 % |
| 9 | Residual Solvents | Must pass test |
Uses
- Silver nitrate is a very versatile compound because the nitrate ion can be replaced by other ligands that can bind to the silver ion. Thus, it acts as a precursor to other silver compounds.
- Silver nitrate is used in many ways in organic synthesis, e.g. for deprotection and oxidations.
- It is used to prepare some silver-based explosives, such as fulminate, azide, or acetylide.
- Silver nitrate is used to demonstrate reticular fibres, proteins, and nucleic acids using silver staining. As a result, it’s also utilised to demonstrate proteins on PAGE gels.
- It can be used as a stain in scanning electron microscopy.
- It is used as an electoral stain.
- When silver nitrate is treated with halide ions, it forms a silver halide precipitate. This property is used in the production of photographic films.
- Fused silver nitrate, shaped into sticks is used as a cauterizing agent.
- When diluted with water to a concentration of 0.5%, silver nitrate can serve as an antiseptic in many medical setups.
Side effects
Acute side effects caused by silver nitrate include:
- It causes irritation due to its corrosive nature. It creates greyish staining of tissues when it is absorbed in the skin over a long period of time (Argyria).
- It will irritate and harm mucosal membranes if inhaled.
- When Silver Nitrate is taken by mouth, it can have significant side effects and could result in death. Vomiting, bloody diarrhoea, dizziness, and seizures are all possible side effects.
Chronic effects due to long term exposure to Silver Nitrate are:
- It can cause general depression, headache, and loss of mental capability.
- Cancer has also been observed in certain people who have been exposed to Silver Nitrate.
FAQs
Q. Is Silver nitrate soluble in water?
Silver nitrate is highly soluble in water with a solubility of about 245 g/100 g of water.
Q. How long does silver nitrate stay on the skin?
If you detect silver nitrate instantly on your skin, you can remove it immediately with a cloth or paper towel, this stain will only last a few days. If your skin is dry, the silver nitrate might take 4 to 5 days to come off the skin.
Q. Is Silver Nitrate Carcinogenic?
Silver Nitrate has not been tested for its ability to cause cancer in animals. Thus, adequate data is not available regarding its carcinogenicity.
Q. How to use silver nitrate sticks?
Silver nitrate sticks come in the form of wooden sticks with 75% silver nitrate and 25% potassium nitrate on the tip. The chemical compounds are activated with moisture either through the application of water or by contacting a moist membrane or wound. The stick should be applied to the wound with a gentle, rolling motion. Two minutes of application time is usually sufficient, but treatment will vary case by case.
Q. What happens when Silver nitrate reacts with Sodium chloride?
Sodium chloride (NaCl) reacts with silver nitrate (AgNO3) to produce silver chloride (AgCl) and sodium nitrate (NaNO3).

