What is Sodium Oxybate?
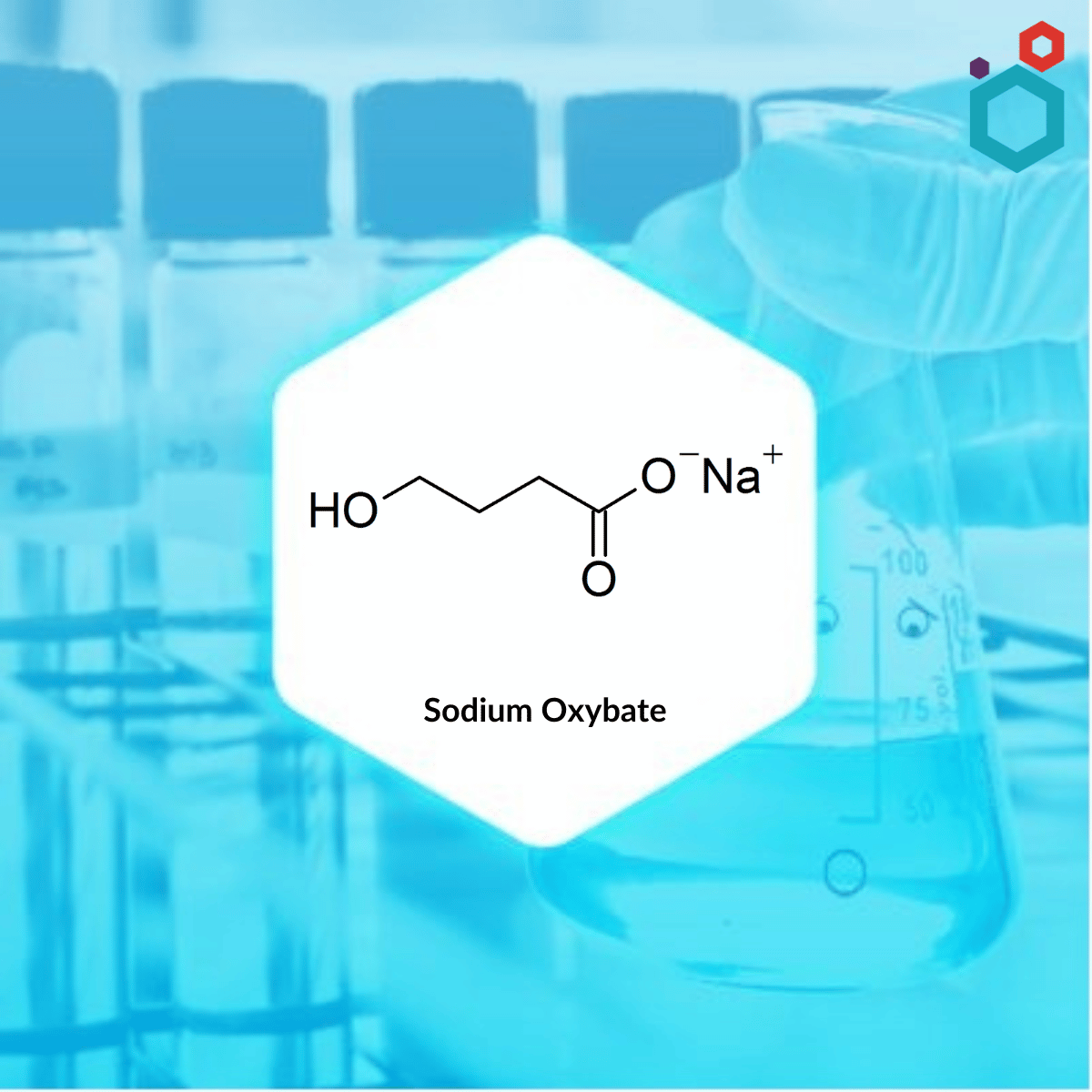
The sodium salt of γ-hydroxybutyric acid (GHB) is called sodium oxybate. It is a medicine that is used to treat symptoms that are associated with narcolepsy such as abrupt muscle weakness as well as excessive daytime sleepiness. Sodium oxybate is offered under the brand name Xyrem, among other brand names.
In 2002, the Food and Drug Administration (FDA) of the United States gave its approval for the use of sodium oxybate to treat the symptoms of narcolepsy. It was approved by European Union in 2005.
| PRODUCT SPECIFICATIONS | |
|---|---|
| Name of Product | Sodium Oxybate |
| IUPAC Name | sodium;4-hydroxybutanoate |
| Synonyms | Sodium 4-hydroxybutyrate; Sodium 4-hydroxybutanoate; Anetamin; Somsanit; 4-Hydroxybutyric acid sodium salt; Gamma OH |
| CAS No | 502-85-2 |
| Molecular Formula | C4H7NaO3 |
| Molecular Weight | 126.09 g/mol |
| Pubchem CID | 23663870 |
| Pubchem SID | 473127230 |
Chemical Properties
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | Appearance (Form) | Powder |
| 2 | Appearance (Colour) | White |
| 3 | Solubility | Clear colorless solution at 50mg/ml in water |
| 4 | Melting Point | 145-146°C |
| 5 | IR Spectrum | Consistent with structure |
Mechanism of Action
Although the precise mechanism of action of sodium oxybate for treating extreme daytime sleepiness (EDS) and cataplexy is not understood, it is hypothesized that the drug’s therapeutic actions are related to the GABA(B) effects on noradrenergic, dopaminergic, and thalamocortical neurons.
Uses
- Sodium oxybate is used to prevent cataplexy (sudden muscle weakness) attacks in adults and children older than 7 years.
- It is also used to treat excessive daytime sleepiness associated with narcolepsy (a condition that affects one’s ability to fall asleep and can result in excessive daytime sleepiness as well as an overwhelming need to sleep at inappropriate times).
- In more recent times, research on its potential utility in the treatment of alcohol withdrawal syndrome has gotten underway. These first appeared in Italy, where researchers also investigated the drug’s potential to treat alcoholism.
- In France and Italy, it is sometimes utilized in the intravenous administration of anaesthetic procedures.
Side effects
Some of the side effects associated with the use of Sodium oxybate are-
- Nausea, Vomiting
- Dizziness, headache
- Stomach pain,
- Diarrhea
- Increased sweating
Potential adverse effects include-
- Respiratory depression
- Seizures
- Coma
FAQs
Q. Is sodium oxybate a controlled substance?
Under the Controlled Substances Act, sodium oxybate was placed in Schedule III as a controlled substance for medicinal use when it was used in accordance with an FDA New Drug Application (NDA) or an Investigational New Drug Application (IND), while Schedule I penalties applied to its misuse.
Q. Where to buy Sodium oxybate?
