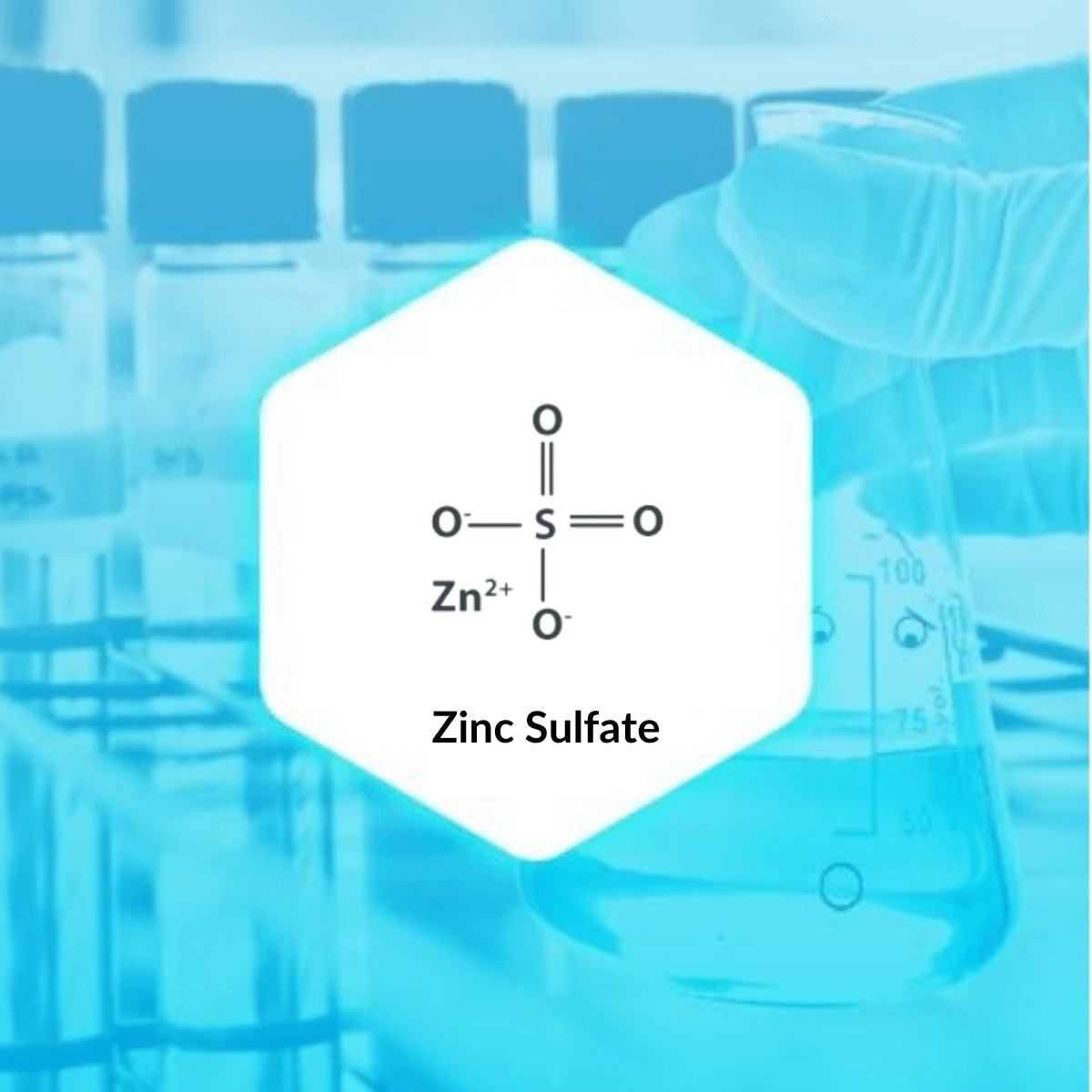
Zinc is a naturally occurring mineral. Zinc Sulfate is an inorganic compound having a combination of sulfur and zinc. It is available as a colourless crystalline solid. The most common form of zinc sulfate is its heptahydrate form having the molecular formula of ZnSO4.7H2O. It was historically known as “white vitriol“.
It is primarily used as a dietary supplement to prevent or treat low levels of zinc as zinc plays a very important role in the growth and development of healthy body tissues. However, excess supplementation may cause abdominal pain, vomiting, headache and tiredness. It is also used as a topical astringent. Products containing Zinc sulfate are also used as pesticides and fertilizers.
The product is currently under development. Samples are available from R&D.
SPECIFICATIONS
| SR. No | Criteria | Limit/Specification |
|---|---|---|
| 1 | Appearance (Color) | Colourless |
| 2 | Appearance (Form) | Crystalline Solid |
| 3 | Solubility (In Water) |
57.7 g/100 mL (20 °C) |
| 4 | Melting Point | 680 °C (decomposes) |
| 5 | Density | 3.54 g/cm3 (anhydrous) | 6 | Uses | Medication used to treat or prevent low levels of zinc alone and together with oral rehydration therapy (ORT) |
