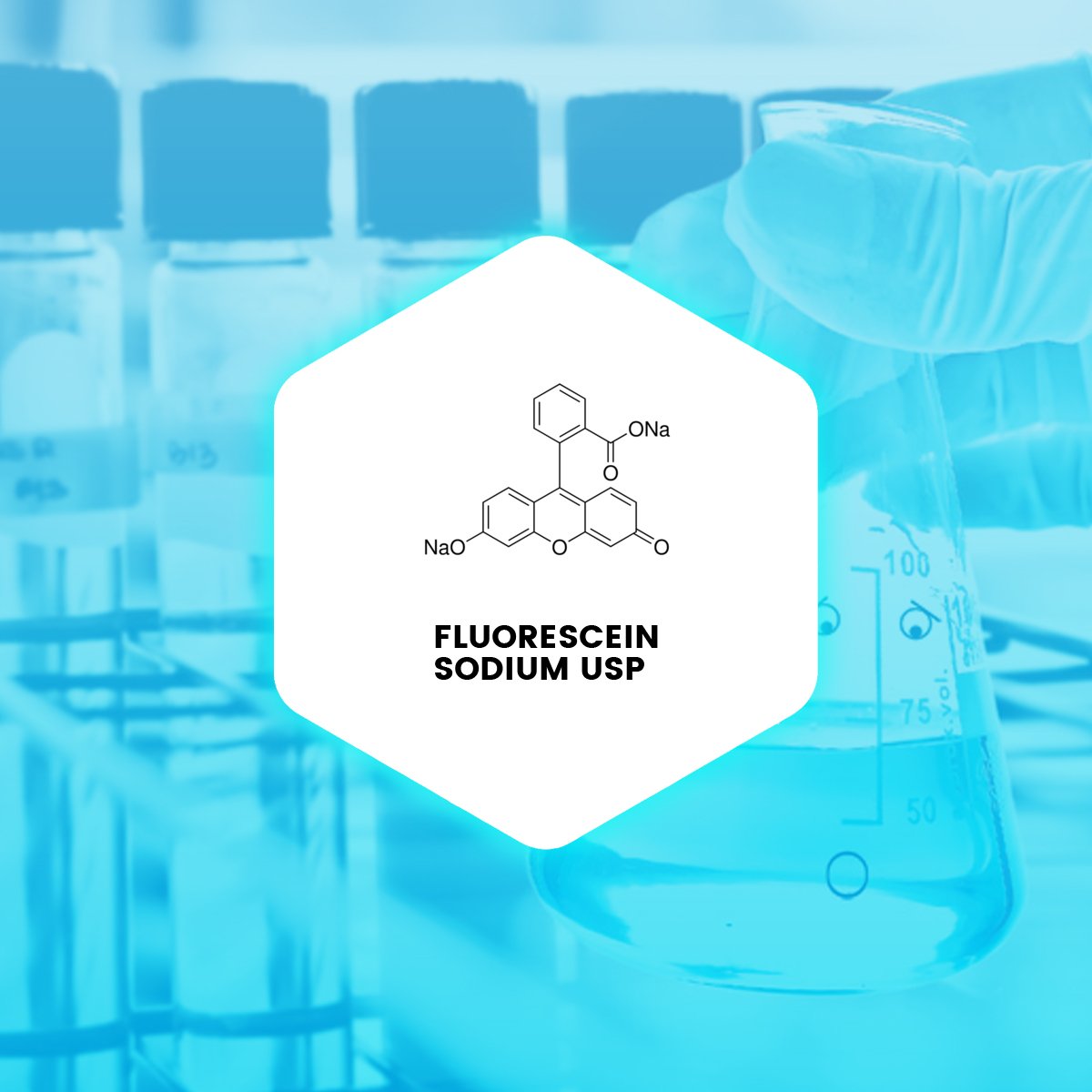
Fluorescein sodium is the sodium salt of fluorescein and a synthetic organic fluorescent dye. It has been used medically in ophthalmology for retinal angiography for many years.
Macsen Drugs granted a patent from Indian Patent office for Novel Fluorescein Sodium Synthesis Process
The Indian patent office has granted a patent to Macsen Drugs, a unit of the Macsen Labs group. The patent relating to the field of chemistry is titled “Process for the preparation of crystalline fluorescein Sodium from diacetylfluorescein or Fluorescein”. The patent concerns a novel process for synthesizing the compound Fluorescein Sodium. Read More.
GMP & ISO Certified Manufacturer of high quality Fluorescein Sodium USP.
Macsen Laboratories, world’s leading manufacturer and supplier of Fluorescein Sodium USP.
Macsen Laboratories, world’s leading manufacturer and supplier of Fluorescein Sodium USP.
SPECIFICATIONS
| SR. No | Criteria | Limit/Specification | Our Results |
|---|---|---|---|
| 1 | APPEARANCE | Orange Red, Fine powder, Hygroscopic | Orange Red, Fine powder, Hygroscopic. |
| 2 | IDENTIFICATION | Must Comply Identification Test A to c | Complies A to C tests |
| 3 | ACRIFLAVINE | No precipitate found | Passes Test |
| 4 | ZINC | No turbidity found | Passes Test |
| 5 | ORGANIC IMPURITIES (HPLC) | 1. RESORCINOL (Impurity A) – NMT 0.5 % 2. PHTHALIC ACID (Impurity B) – NMT 0.5% 3. FLUORESCEIN RELATED COMPOUND C (Impurity C) – NMT 0.5% 4. UNSPECIFIED IMPURITIES (Other Impurity) – NMT 0.1% Unspecified impurity (RT 11.7 Mint.) Unspecified impurity (RT 14.5 Mint.) 5. TOTAL UNSPECIFIED IMPURITIES (Sum of impurities) – NMT 0.5% |
Passes Test Not detected Not detected 0.19 % 0.016% 0.007% 0.023% |
| 6 | WATER DETERMINATION | NMT 17.0% | 8.37% |
| 7 | ASSAY ( On Anhydrous Substance) | 90.0% to 102.0% | 98.79% |
| 8 | Additional test: Pyrogen Free test | Must Pass Test | Passes Test |
RESIDUAL SOLVENTS
| 9 | Acetone | ≤5000 PPM | < 5 PPM |
|---|
MICROBIOLOGICAL QUALITY
| SR. No | Criteria | Limit/Specification | Test Results |
|---|---|---|---|
| 1 | TAMC | ≤100 CFU/g | <10 CFU/g |
| 2 | TYMC | ≤ 50 CFU/g | <10 CFU/g |
| 3 | Bile tolerant Gram-Bacteria | Absent/g | Absent |
| 4 | Staphylococcus Aureus | Absent/g | Absent |
| 5 | Pseudomonas Aeruginosa | Absent/g | Absent |
| 6 | Escherichia Coli | Absent/g | Absent |
| 7 | Salmonella | Absent/g | Absent |
Buy the best quality Fluorescein Sodium USP Used in the field of ophthalmology and used to confirm the presence of residual defect in heart surgery from Macsen Laboratories.
Other Fluorescein Sodium products
For buying, send us an enquiry-
